Which Statement Best Describes the Role of an Irb
Layer 2 also known as the Data Link Layer is the second level in the seven-layer OSI reference model for network protocol design. Which statement best describes the role of an IRB.

Which Statement Best Describes The Polarity Of Cf2i2 In 2022 Molecules Bond Best
The Declaration of Helsinki DoH Finnish.

. It is published weekly. For studies where the only involvement of human subjects is the use of identifiable biospecimens or data originally collected for another purpose complete the PHS Human Subjects and Clinical Trials Information form with information specific to the current study and not the. Parental consent generally is required for the medical evaluation and treatment of minor children.
The Internal Revenue Bulletin is the authoritative instrument of the Commissioner of Internal Revenue for announcing official rulings and procedures of the Internal Revenue Service and for publishing Treasury Decisions Executive Orders Tax Conventions legislation court decisions and other items of general interest. The Internal Revenue Bulletin is the authoritative instrument of the Commissioner of Internal Revenue for announcing official rulings and procedures of the Internal Revenue Service and for publishing Treasury Decisions Executive Orders Tax Conventions legislation court decisions and other items of general interest. Ideally society should strive to facilitate both for the benefit of individuals as well as the public.
Layer2 is the network layer used to transfer data between adjacent network nodes in a wide area network or between nodes on the same local area network. CIO role on program governance boards. Prior to the screening and baseline visits the site SC will verify that the most current IRB-approved study consentassent documents are available for use.
Communicating and Acknowledging Federal Funding. In an NIH National Institutes of Health sponsored study IRB Institutional Review Board chairs reported that complaints about the lack of privacy and confidentiality were among the most common complaints made by. Describes IRB considerations for review of phase I research.
A committee that reviews different types of human subjects research. The report noted that although the actual number of instances in which patient privacy is breached is not fully known. Identifies ways in which researchers and staff involved in phase I research can apply the necessary safeguards to protect subjects including selecting a safe starting dose safeguards for standard dosing regimens selecting appropriate subjects facility safeguards and the role of.
It is published weekly. As noted in the introduction to Chapter 2 the committee views privacy and health research as complementary values. In order to ensure early matching of appropriate IT with program objectives the CIO shall be a member of governance boards that include IT resources including shadow IT or hidden ITsee definitions including bureau Investment Review Boards IRB.
However children and adolescents might require evaluation of and treatment for emergency medical conditions in situations in which a parent or legal guardian is not available to provide consent or conditions under which an adolescent patient might possess the legal. It is widely regarded as the cornerstone document on human research ethics. Which of the following studies most directly contributed to the establishment of the National Research Act and the creation of.
Note for studies involving only the secondary use of identifiable biospecimens or data. The previous chapter reviewed the value of privacy while this chapter examines the value and importance of health research. Several comments said that the statement that an IRB had approved the solicitation of subjects to participate in the research required by proposed 5025a1 could.
Publicizing the outcomes of NIH-funded projects and communicating the role of NIH support in biomedical research improves public understanding of how we the biomedical research community as a whole are working to improve human health. If re-consenting is required throughout the subjects participation in the study the site SC will verify that the most current IRB approved consent is available prior to the study visit. It is not a legally binding instrument under.
Layer 2 is equivalent to the link layer the lowest layer in the TCPIP network model. Helsingforsdeklarationen is a set of ethical principles regarding human experimentation developed originally in 1964 for the medical community by the World Medical Association WMA.

Citi Training Responsible Conduct Rcr Quiz Answers Quizzma

Which Statement Best Describes An Organ Single Celled Organisms Tissue Types Nitrogen Fixation
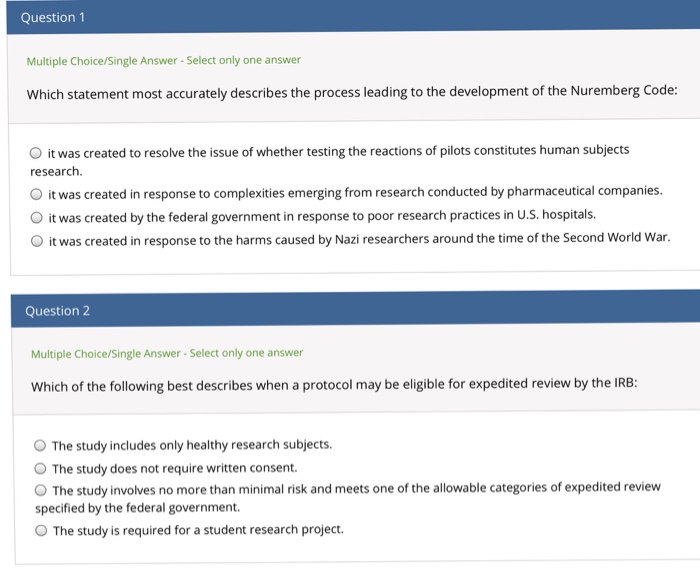
Solved Question 1 Multiple Choice Single Answer Select Only Chegg Com
No comments for "Which Statement Best Describes the Role of an Irb"
Post a Comment